Polysulfone (PSU) is a high-performance engineering thermoplastic in the family of sulfone polymers. Its biocompatibility, high strength, rigidity, dimensional stability, tolerance to repeated cycles in conventional sterilization methods and its availability in both a transparent tint and opaque colors has led to its use in diverse applications in the healthcare industry. The development of an implantable grade commercialized as Eviva® PSU has widened its application potential. Typical areas and applications include:
- Surgical and dental instrument handles and housings
- Mechanical and insulating components for electrical and electronic medical equipment
- Dialysis filtration cartridge housings, caps, and rigid tubing for equipment
- Implantable cardiovascular access ports and drug delivery devices
- Implantable orthopedic bone plates and screws
- Blood oxygenators in cardiopulmonary bypass equipment
- Implantable neurovascular shunts and valves
- Diagnostic imaging for MRI-compatible components
- Dental implant components
- Orthodontic brackets and retainers
- Veterinary medicine applications:
- orthopedic bone plates and screws
- dental extraction systems
- instrument handles
A Closer Look at the Advantages of Polysulfone in Medical Devices
PSU offers an impressive array of performance benefits for medical devices. They include:
- Biocompatibility – PSU as a medical grade plastic has a strong history of success in devices and components that have short-term exposure to internal bodily fluids and tissue. Polysulfone has been used in these applications for decades, as biocompatibility testing shows that the polymer does not produce an immune reaction or noticeable levels of systemic toxicity.
The use of polysulfone in long-term implantable devices is also now occurring with the development of Eviva® PSU, a versatile, high-performance grade that satisfies the requisite ISO biocompatibility and cytotoxicity protocol for these applications
- Resistance to hydrolysis and sterilization –Polysulfone retains a high degree of its initial strength and toughness after numerous autoclave cycles at 121o C (250o F). However, it can degrade after repeated high-temperature autoclaving cycles at the higher 134o C (273o F) level. It also tolerates ethylene oxide gas and gamma sterilization for a limited number of cycles with minimal effect on physical properties.
It should be noted that a similar polymer, PPSU, offers even greater tolerance to sterilization in all conventional methods including high temperature autoclaving.
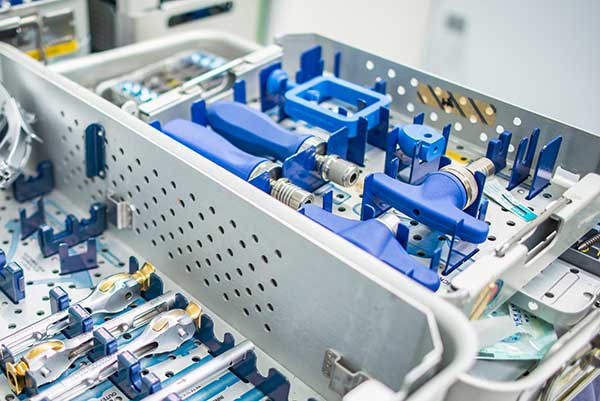
- A robust balance of strength, toughness and dimensional stability –Polysulfone combines high tensile and flexural strength, rigidity and impact resistance with good dimensional stability. Its sterilizability, toughness and rigidity are notable reasons behind its use in surgical instrument handles.
- Resistance to chemicals and cleaners –Polysulfone resists many chemicals, sanitizers and cleaners commonly used in the clinical environment. However, as is the case with any material, variables such as concentration, temperature and physical stress on a device or instrument can affect its ability to retain properties in a given chemical. For this reason, resin suppliers always recommend testing applications under actual conditions of use to validate performance.
How Medical Plastics Processing Specialists Can Support MDMs
Specialists dedicated to high-performance medical polymer injection molding, machinable shapes extrusion and precision machining can provide a number of benefits to MDMs in the development and production of devices and device components in polysulfone and other materials. Among their beneficial roles are:
- Establishing quality, production and logistics processes:During the various stages of medical device development and pre-production, it is essential to establish manufacturing and quality management processes, define conditions for optimizing quality and performance during melt processing, and organize the supply chain. Injection molders and machinable shapes extruders dedicated to medical plastics will have the expertise to support MDMs and facilitate steps in the cycle, from prototyping through finished part production, packaging and logistics.
- Identifying viable material options:Medical grades of polysulfone offer differences in properties to serve a variety of applications. A medical polymer conversion expert has access to a wealth of material and application information both from experience and from close working relationships with their medical plastic resin suppliers. They can be a valuable resource in identifying a streamlined list of candidate materials for evaluation related to the functional requirements of a device.

- Providing prototypes with fast turn-around times:Prototyping is a valuable tool for validating the material candidates and the design of a medical device. Medical plastics injection molders with in-house shapes extrusion and machining capabilities can provide fast turnaround on machined prototypes extruded from stock shapes in a number of materials. Additionally, lower-cost injection molding tooling can be built to produce near-net parts that approximate the final design. They can then be machined to the desired configuration for testing and modified as needed to finalize the specifications.
- Establishing an efficient and reliable supply chain: Major medical polymer conversion specialists typically have close working relationships with leading medical resin suppliers. Both parties often work together on introducing new polymer grades and on developing applications for them. These relationships can support an effective supply chain that begins with reliable scheduling of resin shipments required for on-time production of parts.Working with a medical plastics processing specialist can also take the form of contract manufacturing. In this arrangement, MDMs can hand off the manufacturing and logistics processes to a skilled and reliable partner and focus time and resources on their core competencies in product development, regulatory certifications and marketing.
