The progression from a properties data sheet to a medical device involves several factors that determine how a medical plastic performs in the finished product. The injection molding process as a manufacturing method is an important one, and the factors that optimize it for material performance and device quality are worth the device manufacturer’s consideration.
The Transition from Properties Data to Device Performance

Data sheet comparisons are practical starting points to identify medical plastic candidates for a medical device or its components. From the diverse group of polymers available, device engineers can evaluate medical plastic options and focus in on material candidates that offer an optimum balance of performance, quality, longevity and economics. Once a polymer is selected, engineers then assess such variables as design features, sterilization tolerance and chemical resistance that determine how the plastic performs in the actual device.
These factors are also a good basis for a medical device manufacturer (MDM) to evaluate the capabilities of injection molding companies that are candidates for producing a device.
Guidelines for Evaluating a Plastics Injection Molder for Medical Devices

Manufacturing cost per piece is an important and obvious driver in the selection of an injection molder for a medical thermoplastic device. However, the focus on cost should not override a thorough evaluation of capabilities that can affect the quality and reliability of molded products or components of the device. Those capabilities can in fact provide an advantage in cost efficiency.
The following guidelines cover several of these factors. They can serve as a checklist in qualifying and selecting a capable thermoplastics injection molder for medical devices and components:
- Medical industry quality management systems and certifications: Medical device manufacturers are held to more stringent compliance demands and safety and performance standards than most other high technology industries. A medical plastic injection molder with the appropriate quality management system including full traceability capabilities and ISO certification can be an asset to the device manufacturer in meeting these standards. Ideally, a capable injection molding company serving the industry will be certified to ISO 13485:2016 and can coordinate FDA registration as a device manufacturing facility when required for a medical device project. The facility will also be certified to ISO 9001:2015.
These quality credentials in the manufacturing process make communication between the MDM and the injection molder easier, clear and efficient. They also demonstrate that the injection molder’s operations team understands and maintains the necessary quality protocol as routine standard procedure. - Melt behavior analysis: An injection molder’s ability to analyze a medical plastic’s melt flow characteristics is major asset in designing tooling for the application. An investment in this analytical capability also helps define the optimum melt process conditions in the barrel of the molding machine and in the injection mold. Applying the analysis to processing and tool design can avoid material degradation, optimize properties and yield instruments and precision devices to specified dimensions from cycle to cycle and run to run.
-

The ability to upgrade and maintain tooling transferred from another facility solidifies the working relationship between an MDM and their preferred injection molder. Tool building and maintenance: Once the tolerances and other specifications for a part or device are defined, engineers familiar with the material specified for the application and its flow characteristics can help develop the optimum tool design. In-house tool maintenance is also a beneficial service, as is the ability to manage a tool transfer from another injection molder should circumstances create the requirement.
-
Dedicated dryers: Drying medical plastics at temperatures and for durations specific to a material is a routine practice among injection molders. However, an added and worthwhile quality step involves an investment in dryers dedicated to the conditions required for each specific medical polymer. This avoids cross-contamination with other materials, which can adversely affect the quality and performance of the finished part.
-

Clean, resin-dedicated dryers in a closed-loop material handing system support consistent quality in injection molded devices. Contained material handling: A closed, controlled system for transferring the pelletized medical plastic resin from shipping containers to dryers, then from dryers to the molding machine hopper minimizes the risk of contamination from airborne particulates. A robust transfer system also maintains the right level of material dryness in the polymer through each phase.
-
Post-production tear-down and cleaning: Another potential source of contamination can be residue from prior production runs. Thermally degraded material can affect performance of the specified polymer. It can also create unacceptable black specks and burn marks on the surface of a medical device.
Avoiding these quality problems requires a full tear-down and clean-up of all parts of the molding machine that contact polymer melt after each production run, including the screw and molds. -
Versatility to produce different configurations: An injection molder with machines in a range of tonnages offers several benefits to a device manufacturer. For example, a single source for component parts of significantly different sizes or for different sized devices within a kit simplifies scheduling and logistics. A diverse equipment capability also facilitates the transition to production of new devices that differ in size and complexity.
-
- Electric (non-hydraulic) injection molding machines: All-electric injection molding machines eliminate the risk of contamination from hydraulic fluid. The contamination can occur inadvertently to pelletized material, or to parts after they are ejected from the tool.
Injection-compression technology is also an asset that expands an MDM’s design and engineering possibilities. The capability minimizes the risk of voids that can occur in devices with relatively thick cross-sections.
Overall, an injection molder’s ability to meet production requirements for a variety of injection molding applications and designs fosters a long-term relationship that streamlines communication, scheduling, planning, technical support and fast turn-around when the need arises. -
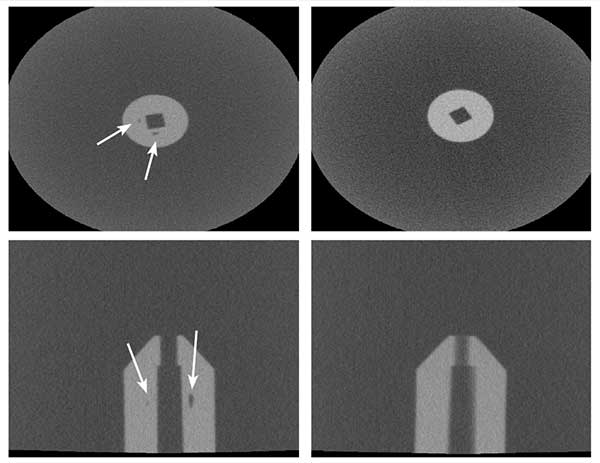
After ongoing quality issues with another supplier, the MDM’s preferred injection molder ran CT scans of rejected implantable PEEK devices. They resolved voids evident in the left scans using injection-compression technology. A well-equipped analytical and test lab: State-of-the art analytical and test equipment in the hands of skilled technicians are indispensable tools for quality management in medical device injection molding. Prior to production, a well-equipped lab can also analyze melt characteristics with the accuracy needed to define optimum processing and in-mold conditions for the medical plastic that is specified.
Additionally, while outsourcing may be an option for analytical testing, it can involve lead-times that can hold up production or development projects when problems occur. An injection molder with the technical experience and the right equipment can prioritize the analytical work and testing services needed to develop injection molding solutions that restore production quickly.
Capabilities of a well-equipped in-house lab for quality management and analytical work include:
-
- Universal ASTM test apparatus to validate physical properties of materials and parts
- Non-destructive parts testing by ultrasonic and CT (computer tomography) to detect potential voids in thick cross-sections of parts
- DSC (differential scanning calorimeter) to characterize thermal properties
- Resin melt-flow analysis equipment to define optimal process and in-mold melt conditions and tool design
- In-house machining: Machining is another service that can be outsourced. However, with in-house CNC machining centers, an injection molder can quickly produce prototypes. Prototyping is particularly useful for validating design and material changes that need to be translated to injection molding tooling.
Machining can also add complex features to injection molded parts that are beyond the limits of the molding process or are too costly to incorporate in tooling. An in-house capability incorporates the finish-machining operation within an efficient production cycle.
Technical Expertise Completes the List
A well-experienced technical team rounds out the attributes of a capable medical plastics injection molder. Engineers with a depth of hands-on experience will achieve the full benefit from sophisticated injection molding equipment, control systems and tooling. Talented process engineers can also adapt and modify off-the-shelf injection molding equipment and control systems to raise the bar on quality and consistency for their medical device customers.
